Throughout the pandemic, the branch of medical equipment and diagnostics has made a great contribution to the fight against the Kovid-19. According to Worldometer.info, more than 64 million cases of the disease are currently registered in the world, as a result of which 1.5 million people died.
In this review we will present 10 major achievements in the field of medical equipment in the fight against the Kovid-19.
1. Healthcare becomes virtual and finally begins to take telemedicine

Telemedicine in recent years slowly and gradually became increasingly familiar in the health care system. Pandemic Cowid-19 accelerated this process for five years or so. As the virus pushed society to virtual business, telemedicine became a natural solution for patients and doctors. No longer needed to wait in the clinics, ordinary medical examinations it became possible to spend straight from the patient's apartment.
Telemedicine really went to the fore and, according to forecasts, will have a strong presence and at the end of the pandemic.
2. Quick Test for Cowid 19

Guardant Health is already known for its tests for the "liquid biopsy" in order to identify cancer. Nevertheless, this American company produced a furor in the field of medical technologies, when in August received a formal permission to emergency use its test for the detection of Cowid-19.
According to the representatives of Guardant, the COVID-19 test was then used in order to help the University of Delaware's university safely resume work. The test was also used to verify the health of health workers Guardant Health and selected partner organizations through a certified company laboratory.
According to the Statement Guardant Health, the development of the test for the definition of the CABID-19 was its "civil debt", and in no case refused to develop tests on cancer.
3. Joint work of technological giants

Google and Apple have always been fierce rivals, but the virus has created a situation that demanded that they work together. In May, technological rivals jointly launched the Exposure Notification technology, which is designed to inform someone about possible infection, if he came into contact with the person who was diagnosed with the CAID 19. Such warnings could receive people who enjoyed iOS or Android-based smartphones.
In his joint release, "temporary partners" said:
What we have created is not an application - rather this API that public health organizations will be able to include in their applications to install users. Our technology is designed to make these applications better. Each user can decide independently, subscribe or not on the Exposure Notification service, while the system does not collect and does not use location data from the device. And if a person is diagnosed with Cossout 19, he can decide himself, report this in an application for public health or not. The key to success is to adapt such a service by users, and we believe that strong privacy protection tools used here are also the best way to encourage these applications.
4. Devices for the control of cardiovascular activities began to influence the treatment of patients with CABID-19
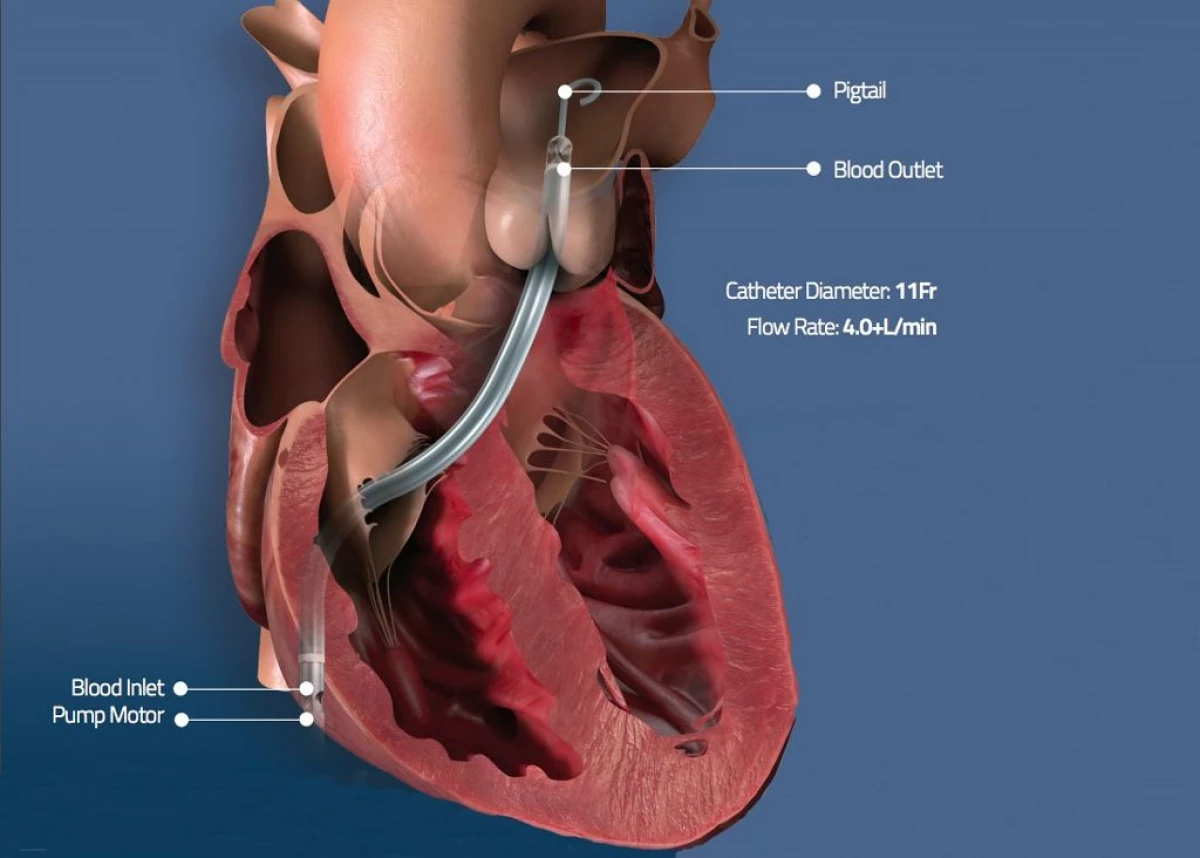
AbiMed was one of the first organizations in the Cardiovascular Health Control Systems, which attracted attention and received permission to emergency use its technology to assist in the treatment of Sovid-19 symptoms.
This company in June 2020 received a formal permission to use its IMPELLA RP device, which is a miniature catheter cardiac pump for oxygenation therapy while reducing the load on heart muscles in cases of life threatening, due to head unloading and ensuring mechanical blood circulation support.
Now this system has found its application in the case of COVID-19 in patients suffering from right-hand heart failure, including blood closures blocking blood flow into the lungs.
Impella's cardiac pump helps the heart pumping blood to the aorta - the main artery that is bored with oxygen blood from the heart to the body, and is intended to support patients with COVID-19, which pass therapy using extracorporeal membrane oxygenation and which develops lungs or myocardine swelling.
According to the doctor of medicine Sean Chakrabarti (Shon Chakrabarti) from the Medical Department of AbiMed,
We definitely see the vascular effect in patients with Cowid-19. We observe that this disease causes a protadotic medium in some patients. Some of them have an acute pulmonary embolism and our device can potentially save many lives.
The application for the use of the AbiMed apparatus was all the more significant because it shed light to potential damage, which can apply the heart of the European Union.
5. Sharp growth of digital health financing

Attracting venture capital at the beginning of the global pandemic was a significant problem for many medical devices manufacturers. But for companies dealing with digital health care, the situation looked completely different. In June, the American analytical company MERCOM CAPITAL GROUP reported that the volume of investments in digital health care in the first half of 2020 was 24% more than in the first half of 2019, when $ 5.1 billion came to the market. And this despite The fact that the pandemic caused a heavy blow to the global economy.
The sharp leap of funding was caused by the wider introduction of digital technologies and products during the outbreak of CABID-19, Mercom specialists noted.
The most funded categories of digital health care in the first half of 2020 were the following:
- Telemedicine - $ 1.9 billion;
- Analytics - $ 826 million;
- Mhealth applications - $ 794 million;
- Clinical solutions support systems - $ 545 million;
- Doctor Reservation Systems - $ 325 million;
- Wearing devices - $ 321 million.
6. Medtronic lay out in open access design documentation of its device for artificial ventilation of the lungs
At the beginning of the pandemic, concerns increased due to the lack of artificial ventilation devices in the health market. This led to the fact that even many companies from the automotive industry began the development and production of such breathing apparatus. This took up even a manufacturer of bracelets for fitness FITBIT, which received permission to emergency use its fan.
Medtronic also responded to this challenge and publicly shared design specifications on one of the models of his device for artificial ventilation of the lungs - Puritan Bennett 560 (PB 560). This step allowed companies operating in different industries, evaluate options for the rapid fan production in order to satisfy the sharp need for more such devices for patients with CABID 19.
7. Five-minute ABBOTT Test

Abbott Laboratories has repeatedly raised the interest of a professional press due to its COVID-19 tests. Nevertheless, the test sets of the company became the main topic of the discussion when Binaxnow COVID-19 AG Card was presented - the test for the antigen-19 antigen, allowing the result in 5-15 minutes. The company received a formal permission to use this test in August 2020.
Due to the affordable price and guaranteed rapid result, the test was recognized as changing the rules of the game in this area.
8. Food and Medication Quality Control Office (FDA) now does not regulate the use of laboratory tests.
August has become a turning point in the USA in the regulation of tests on the Kovid-19 and laboratory tests in general.The US Department of Health and Social Services reported that, in his opinion, the Food and Drug Administration, Food and Drug Administration, FDA (US Food and Drug Administration, FDA) should not require prior approval of test laboratories in mind the lack of clear rules necessary for obtaining Permits of this state department.
Further, the report states that "persons requesting approval or permission to extradite tests may, nevertheless, voluntarily submit an application for preliminary approval, but they are not obliged to do this, and the FDA will decide on these applications."
According to the material published in The Washington Post, such a ministry policy has become a shock for the FDA.
9. Standardization of antibody tests
In the market, there was a confusion with tests for antibodies. They are different from different test manufacturers and are currently not analyzed and compared, as they are aimed at various SARS-COV-2 virus proteins. To solve this problem, Siemens Healthineers united with the Centers for the Control and Prevention of US Diseases, as well as with the United Research Center for the European Commission to standardize tests.
As part of this project, the German company is developing the standardization process of SARS-COV-2 analyzes by binding each protein to the titer of the neutralizing antibody - the level of the antibody present blocking the penetration of the virus into the cells during laboratory experiments. The threshold values displayed in a standardized unit of measurement for IgG antibodies that appear either from natural infection, or from vaccination, may probably contribute to standardized interpretation of immunity through test results.
10. The American regulator has changed its strategy of his actions.
At the end of January 2020, United States (FDA) products and medicine control facilities developed a strategy for the fight against ACSID-19, which we observe in action today in the United States and some other countries. Part of this strategy was the use of the method of authorization of the application of medical solutions in an emergency (Emergency Use Authorization), which arose due to a pandemic. FDA stated that it would work with interdepartmental product development partners, international partners and global regulators to speed up the development and availability of medical products necessary for diagnosis, treatment, softening and preventing outbreaks of the epidemic.
When the FDA presented its strategy was recorded by a little more than 4,000 cases of virus infections that were confirmed in China. More than 100 people became victims of this disease - however, at that time no man did not die in the United States. Such a quick response of the state department has become an example of organizing actions for many countries.
According to Medtech Innovations, MDDI Online, Mobihealth News.
